Novo Nordisk Foundation Cellerator
The initiative aims to advance the cell therapy ecosystem and enable transformative treatments.
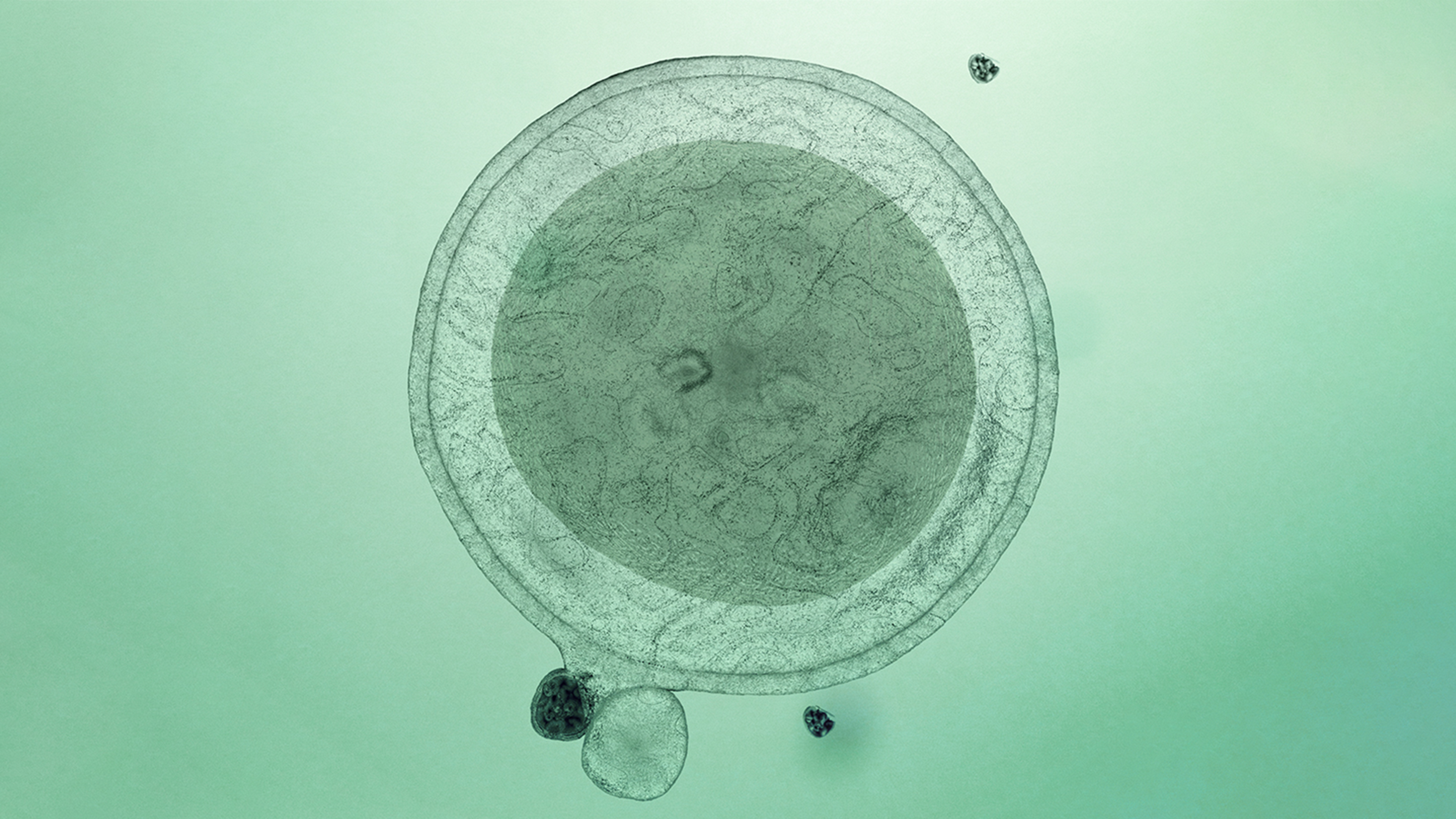
Cell therapy is a rapidly advancing field that involves transplanting healthy, living cells to restore damaged tissues and organs. These therapies make it possible to treat diseases themselves, not just symptoms, and potentially even to cure chronic or life-threatening diseases such as Parkinson’s, kidney disease, type 1 diabetes, chronic heart failure and some forms of cancer.
But despite major advances in research, many promising cell therapy candidates do not reach clinical trials, partly because of a lack of facilities that can produce cell therapy products in large, consistent quantities.
The Novo Nordisk Foundation Cellerator will help fill this gap. The primary activities at the state-of-the-art laboratory facility will be further developing cell therapies that have already been tested successfully in animals, and manufacturing these consistently and at scale for early clinical trials.
The facility will support several cell therapy types – those derived from induced pluripotent stem cells, from embryonic stem cells and from adult stem cells – and provide a range of services, from process development to product GMP manufacturing, product release and regulatory support. There will be built-in flexibility with regard to therapy types and services in order to respond to changing demands in a field that is developing rapidly and currently is highly explorative.
The Foundation is already active in the cell therapy field through the Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW, where pioneering research is driving the development of new treatments. The Novo Nordisk Foundation Cellerator will help ensure successful treatments developed in laboratories at reNEW and elsewhere can reach clinical trials.
The facility will be located at the Technical University of Denmark (DTU) in Lyngby, north of Copenhagen. The Cellerator team will collaborate closely with DTU in both the construction and the operational phases, drawing on the university’s significant expertise in cell manufacturing and related technologies, building synergies across research and education, and engaging with DTU’s innovation ecosystem, including start-ups.
The Cellerator will serve public and private, national and international clients from academia, biotech and the pharmaceutical industry. Approval will be sought from the Danish Medicines Agency to produce cell therapies for clinical use and the facility is expected to be operational in 2027.
Until then, the Novo Nordisk Foundation Cellerator team, headed by CEO Thomas H.R. Carlsen, is working to build links with researchers and other cell therapy development and manufacturing facilities. Project Director Birgitte Fauerholm Saabye at the Novo Nordisk Foundation is leading on the implementation of the facility, including design, construction and equipping.
The Cellerator will be established as a limited liability company fully owned and funded by the Novo Nordisk Foundation. Any profits will be reinvested in the facility.
Read more here.
